The Future of Fashion: Exploring the 3D Fashion Design Software Market
September 25, 2025 | News | No Comments
The Future of Fashion: Exploring the 3D Fashion Design Software Market
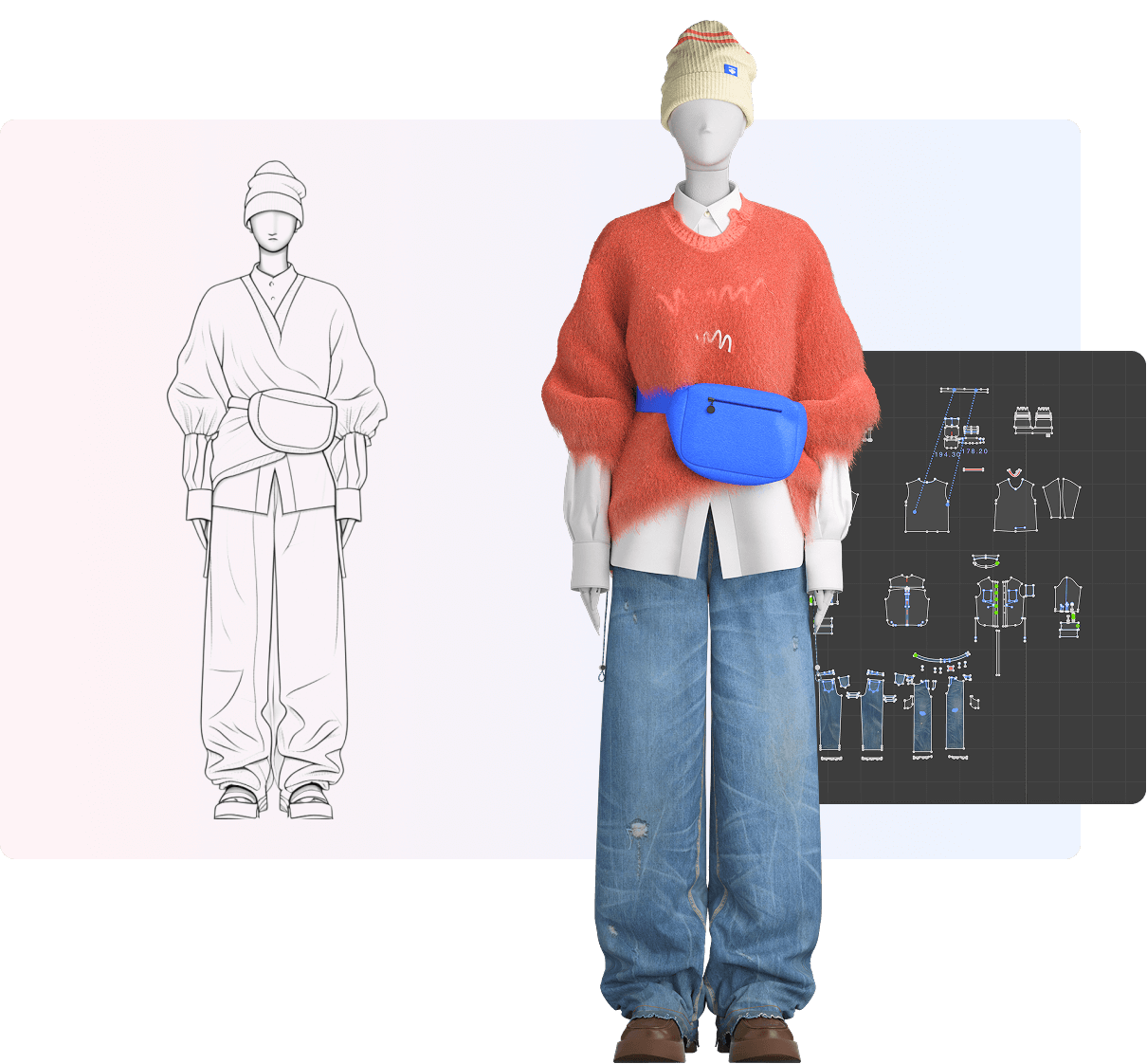
The fashion industry is undergoing a digital revolution, and at the heart of this transformation is the rapidly evolving 3d fashion design software market. This technology is reshaping how designers create, prototype, and bring their visions to life, moving beyond traditional 2D sketches into dynamic, photorealistic 3D environments.
Key Drivers of the 3D Fashion Design Software Market
The growth of this market is fueled by several key factors. The demand for faster production cycles and the rise of digital sampling are reducing waste and costs significantly. Furthermore, the integration of AI and VR is pushing the boundaries of what’s possible, enabling hyper-realistic simulations and personalized shopping experiences.
Digital Prototyping and Sustainability
A core advantage of 3D fashion software is digital prototyping. Designers can create accurate virtual samples, allowing for endless iterations without physical materials. This not only speeds up the design process but also aligns with the growing industry emphasis on sustainable fashion practices by minimizing textile waste.
Frequently Asked Questions
What is 3D fashion design software?
It is specialized technology that allows fashion professionals to design, visualize, and simulate garments in a three-dimensional space, creating realistic digital twins of physical clothing.
How does 3D design benefit the fashion industry?
It streamlines the entire product development process, from initial concept to production, reducing time-to-market, cutting sampling costs, and enhancing collaboration between global teams.
Embrace the Digital Shift
The future of fashion is undeniably digital. To stay competitive, brands must adopt these innovative tools. Explore the powerful solutions available in the 3d fashion design software market to revolutionize your design workflow.
Ready to transform your design process? Discover how leading software can unlock new levels of creativity and efficiency for your brand.